As companies hunker down for the effects of the COVID-19 virus, they are abandoning their IP protection at TWICE the normal rate.
And it is likely to go up much further. AND this has never happened before.
BlueIron’s study looked at recent USPTO abandonments to see what, if any, effects were from COVID-19. The results were enlightening.
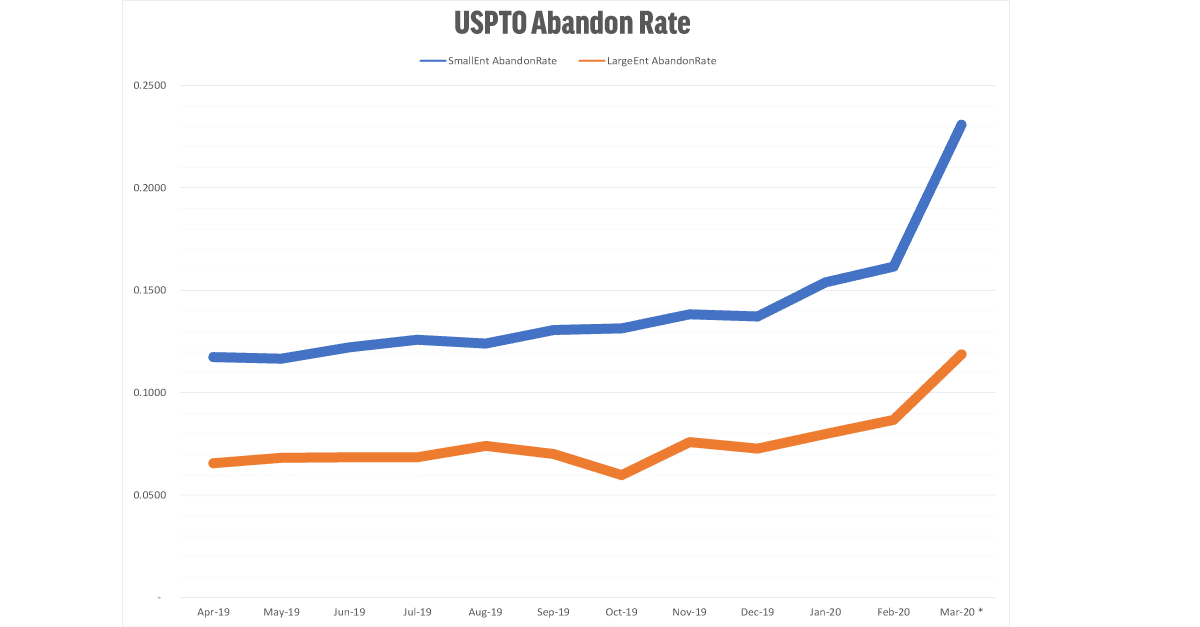
For both Small Entities (under 500 employees) and Large Entities, the normal rate of abandonment is about 12.5% and 7.5% (respectively). In March 2020, the abandonment rate has nearly doubled to 23% and 12%. (For example, small entities received 5747 office actions in September, and 1326 were abandoned – a 23% fall off rate.) The data from April 2020 is expected to be even higher.
Patent protection is a very long term play – the assets last 20 years. In some respects, patents reflect the company’s optimism and commitment to the future. Why would a company get a patent? Because they plan on being in business to use that asset during its 20 year life span.
Companies abandon patent applications for many reasons. Sometimes, the examiner finds prior art that kills the patent dead in its tracks, but most often it is a cost issue. Companies will continue fighting with the examiner and investing money into their patent because they see value, even if they have to amend their claims.
The patent value equation changed dramatically in the last couple months.
Companies are twice as likely to tell their patent counsel to abandon their patent applications lately, compared to historical averages.
For most companies, in my opinion, this reflects possibly two factors: the need to conserve cash and, more worrisome, possibly more pessimism about the future. A third option is that entrepreneurs are happy to have an excuse to cut a pointless expense.
Cash Conservation
Abandoning a patent application certainly conserves cash in the short run. The US average cost to pay an attorney to file a response to an examiner’s rejection is $4000. The raw data are available here from AIPLA (membership required). In a typical cadence, each patent application will require two to three responses filed per year, so keeping a patent application alive saves $8-12K/year in direct cash outlays.
I do not believe this is cash conservation. This is something different – and much more dangerous.
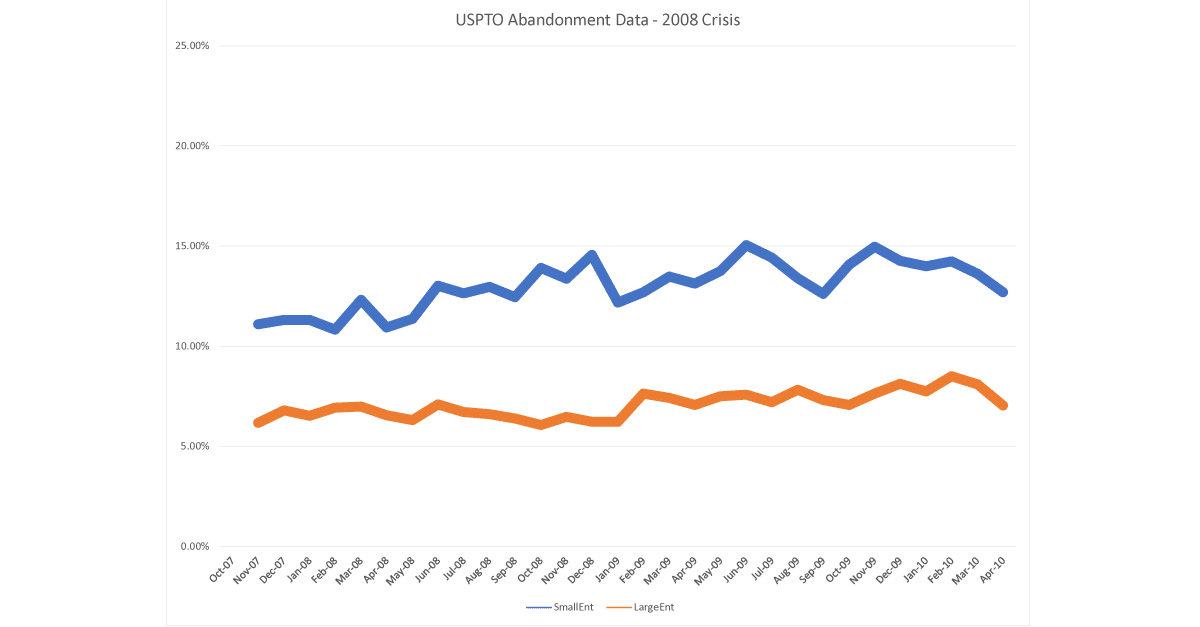
This reaction did not happen in 2008, during our last stock market crash and reset. Throughout all of that period, abandonments went up and down, but hovered at 12% for small entities and 6-7% for large. This is the same rate for most of 2019.
Then something changed – and it changed in a way we have never seen before.
I believe this change is a severe pessimism on all fronts.
Pessimism About The Future
Patents are inherently forward-looking. They are bets that a company’s technologies will be valuable in the future, and, for all practical purposes, patents are call options on technology.
Patents are inherently optimistic. The investment reflects the inventor’s belief that they will be successful in bringing their product to market, and competitors will pop up. Patents are the Big Stick that will protect the entrepreneur’s investment in their business.
Abandoning patents can signal that business owners are not as optimistic as they once were about their ideas. This signal could indicate that even the entrepreneur does not believe in their own ideas, and might be a leading indicator that the entrepreneur will pivot or even close down the enterprise.
Dry Powder
Previous economic downcycles have dumped lots of IP on the open market. In fact, the “industrialization” of IP occurred right after the dot-com era. Intellectual Ventures was formed out of the dust of the dot-com bubble. The term “patent trolls” was born from people trying to generate value from IP left over from the failed startups of that time.
If patents were valuable before the Corona virus, they will be even more valuable after. With companies abandoning their IP, this means that those that keep their IP alive will have an even better competitive advantage when the inevitable recovery happens. Quite simply, they will have IP protection when their competitors do not.
What does this mean for USPTO applicants? It means better, faster examination for those who keep their applications pending.
What does it mean for patent attorneys? The gravy train of patent prosecution is likely to dry up, and it will take years for the pipeline to be refilled with new applications. I don’t think there will be many tears shed for the fate of the patent attorneys.
About the graph:
The graph shows the abandon rate for small entities (less than 500 employees) and large entities. Universities, non-profits, and independent inventors are removed from this dataset.
USPTO rejections allow 6 months to respond. The labels reflect the last date available for response. In other words, the data labeled March 2020 reflect final and non-final rejections issued by the USPTO during September 2019. Note that March 2020 contains data for the first three weeks of March only.
The patent abandonment data are a lagging indicator. We do not see the effects of a decision to abandon a patent application until after the six month window to respond has ended. By all indicators, the data for April 2020 will be much worse than March 2020.
The raw data were compiled from AcclaimIP.
About the Author :
Russ Krajec is an accomplished author, inventor, and engineer. Russ is the author of “Investing In Patents” and the forth-coming book “Startup IP Strategy,” as well as countless blogs and articles. He is a registered patent attorney, having drafted nearly 1000 patent applications since passing the patent bar exam in 2000. Russ is a Certified Patent Valuation Analyst.
He is the CEO of BlueIron, which finances patents for startup companies. BlueIron finances pre-revenue startup companies with $50-100K investments to build out their patent portfolios. For post-revenue companies, can do loans of $2-5M, using only your patents as collateral.
Russ holds a BS and MS in Mechanical Engineering from Rensselaer Polytechnic Institute and a JD from Denver University.
If you wish to speak to him for short call or analysis of your Patent related query, you can schedule an online meeting by clicking here. Alternatively, you can send an email on [email protected] with copy to [email protected].
 Online Enquiry
Online Enquiry
 Useful Links
Useful Links